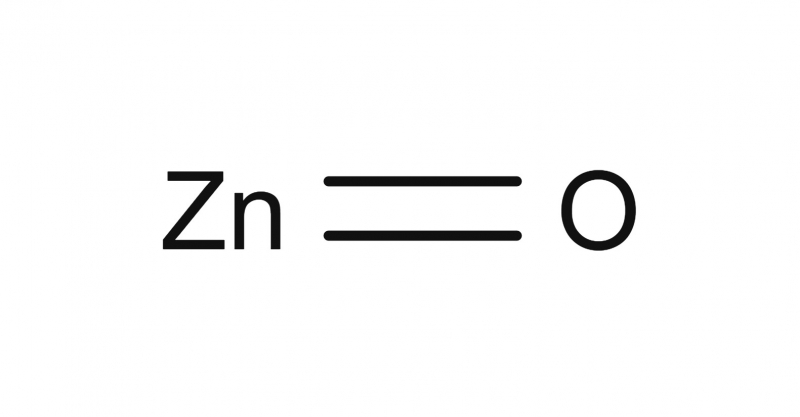Zinc (II) oxide, Pharma
ZnO
Quality
Pharma grade, obtained by the indirect (French) process quality according to USP, EP, BP with a purity of ca. 99.8% or as pharmaceutical GMP goods to the standard GMP1, GMP2, GMP3.
Description
Typically, a fine, loose and - due to the refraction of light - white powder which is practically insoluble in water.
In nature, it occurs as a mineral Zincite, with the majority of the zinc oxide used is synthetically prepared.
As pharmaceutical quality, it can be found in many different applications of care and Healthcare products (eg sunscreen, skin diseases / wound healing).
Packaging
25 kg bags
Storage
Store dry.
Availability
Minimum: 1000 kg
| Details | |||
|---|---|---|---|
| Name: | Zinc oxide, pharma | ||
| EC-name: | zinc oxide | ||
| IUPAC Name: | oxozinc | ||
| Chemical Formula: | ZnO | ||
| Molecular Forma: | OZn | ||
| CAS-No.: | 1314-13-2 | ||
| EC-No.: | 215-222-5 | ||
| Hazard statements: |
GHS:
Attention
GHS09
UN-No.: 3077 Transport Class: 9 Packaging Group: III |
||